CJC-1295 vs Sermorelin
CJC-1295 and sermorelin are both GHRH analogs that stimulate pituitary growth hormone release, but they represent different generations of peptide engineering. Sermorelin is bioidentical GHRH(1-29) — the minimal active fragment of native GHRH — with decades of clinical history and a former FDA approval. CJC-1295 is a modified GHRH analog with amino acid substitutions for protease resistance, and its DAC (Drug Affinity Complex) variant extends the half-life from minutes to approximately 8 days by binding serum albumin. This pharmacokinetic difference is the key distinction: CJC-1295 allows less frequent dosing and produces more sustained GH/IGF-1 elevation, while sermorelin creates shorter, more physiologic GH pulses.
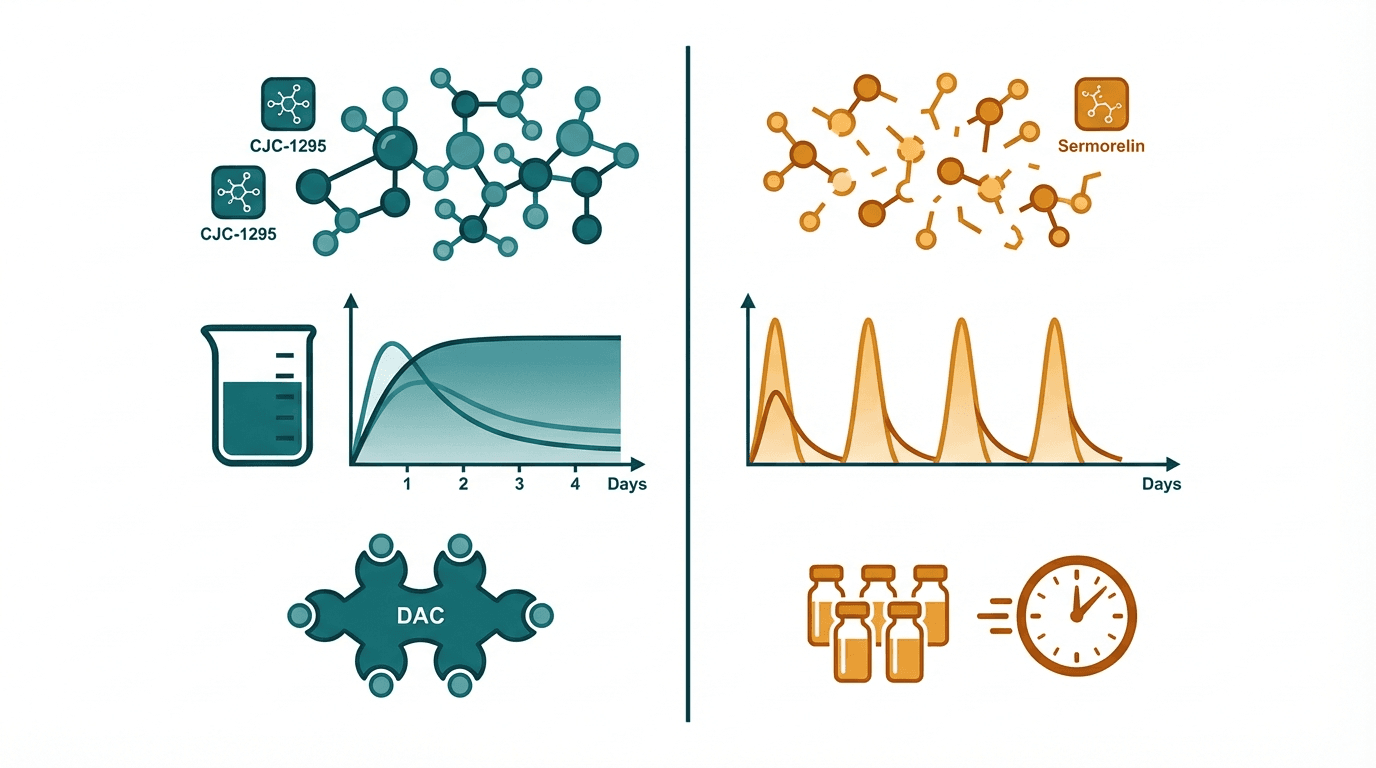
Head-to-Head Comparison
| Criteria | CJC-1295 | Sermorelin |
|---|---|---|
| Primary mechanism | GHRH receptor agonist — modified GHRH analog with enhanced stability | GHRH receptor agonist — bioidentical GHRH(1-29) fragment |
| Amino acid modifications | 4 amino acid substitutions (Ala2, Gln8, Ala15, Leu27) for protease resistance; optional DAC conjugation | None — identical to first 29 amino acids of native human GHRH |
| Half-life | ~30 minutes (no DAC / Mod GRF 1-29); ~8 days (with DAC) | ~10–20 minutes |
| Dosing frequency | 1–3× daily (no DAC); 1–2× weekly (with DAC) | Once daily before bed (standard protocol) |
| Typical dosage | 100 mcg (no DAC) per injection; 2 mg (with DAC) per weekly injection | 200–300 mcg subcutaneous per injection |
| GH release pattern | Pulsatile (no DAC) or sustained elevation (with DAC) | Short pulsatile GH release that closely mimics natural GHRH signaling |
| IGF-1 elevation | Significant and sustained — CJC-1295 DAC shown to raise IGF-1 by 65–115% in clinical trials | Moderate and transient — IGF-1 increases during active treatment, normalizes quickly after discontinuation |
| FDA history | No FDA approval; investigational compound with clinical trial data | Previously FDA-approved (Geref) for pediatric GH deficiency diagnostics; brand discontinued |
| Clinical data depth | Moderate — Phase I/II trials demonstrating GH/IGF-1 elevation and safety | Extensive — decades of clinical use, multiple published studies, former FDA-approved drug |
| Side effects | Injection site irritation, water retention, facial flushing, transient numbness/tingling; DAC version may cause prolonged side effects due to long half-life | Mild — injection site reactions, facial flushing, headache; effects resolve quickly due to short half-life |
| Ease of course correction | No DAC: quick clearance allows easy adjustment. With DAC: 8-day half-life means side effects persist if they occur | Excellent — 10–20 minute half-life means any adverse effects resolve very rapidly |
| Approximate monthly cost | $50–$100 (research grade); $200–$450 (clinic) | $50–$100 (compounding); $200–$400 (clinic) |
When to Choose Each
Choose CJC-1295
Users who want less frequent dosing (especially DAC variant), those seeking maximum sustained IGF-1 elevation, protocols where dosing compliance is a concern, experienced peptide users comfortable with longer-acting compounds
Choose Sermorelin
First-time peptide users who want the most conservative option, physician-supervised protocols that prioritize clinical history and safety data, users who want rapid clearance for easy dose adjustment, those who prefer bioidentical compounds
Verdict
For users who want the convenience of less frequent dosing and maximum sustained GH/IGF-1 elevation, CJC-1295 with DAC is the superior choice — one or two injections per week versus daily dosing. For users who prioritize the most natural GH release pattern, a well-characterized safety profile, and easy course correction if issues arise, sermorelin is the safer and more conservative option with decades of clinical backing. In practice, many clinics have moved toward CJC-1295 (without DAC, also known as Mod GRF 1-29) as the preferred GHRH component in stacks with ipamorelin, as it offers better stability than sermorelin without the extended-release concerns of the DAC variant.
References
- Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults (2006) — PubMed
- The effect of CJC-1295, a synthetic GHRH analog, on pulsatile GH secretion (2006) — PubMed
- Sermorelin: a review of its use in the diagnosis and treatment of children with idiopathic growth hormone deficiency (1999) — PubMed
- Growth hormone-releasing hormone: clinical studies and therapeutic aspects (1988) — PubMed
- Pharmacokinetics and pharmacodynamics of modified GRF(1-29) and its DAC-conjugated analog (2006) — PubMed
Frequently Asked Questions
What is Mod GRF 1-29 and how does it relate to CJC-1295?
Should I choose CJC-1295 with DAC or without DAC?
Is sermorelin outdated compared to CJC-1295?
Can I switch from sermorelin to CJC-1295 mid-protocol?
Do CJC-1295 or sermorelin require refrigeration and special storage?
Explore next
- CJC-1295 dosage guideEducational reference for CJC-1295 dosage protocols with and without Drug Affinity Complex (DAC). Covers modified GRF 1-29, CJC-1295 DAC, and combination stacking approaches discussed in research literature.
- Sermorelin dosage guideComplete sermorelin dosage guide with injection protocols, bedtime timing rationale, 5-on/2-off cycling strategy, reconstitution instructions, and stacking considerations. Covers standard and higher dose protocols based on published research and clinical use patterns.