Retatrutide vs Liraglutide
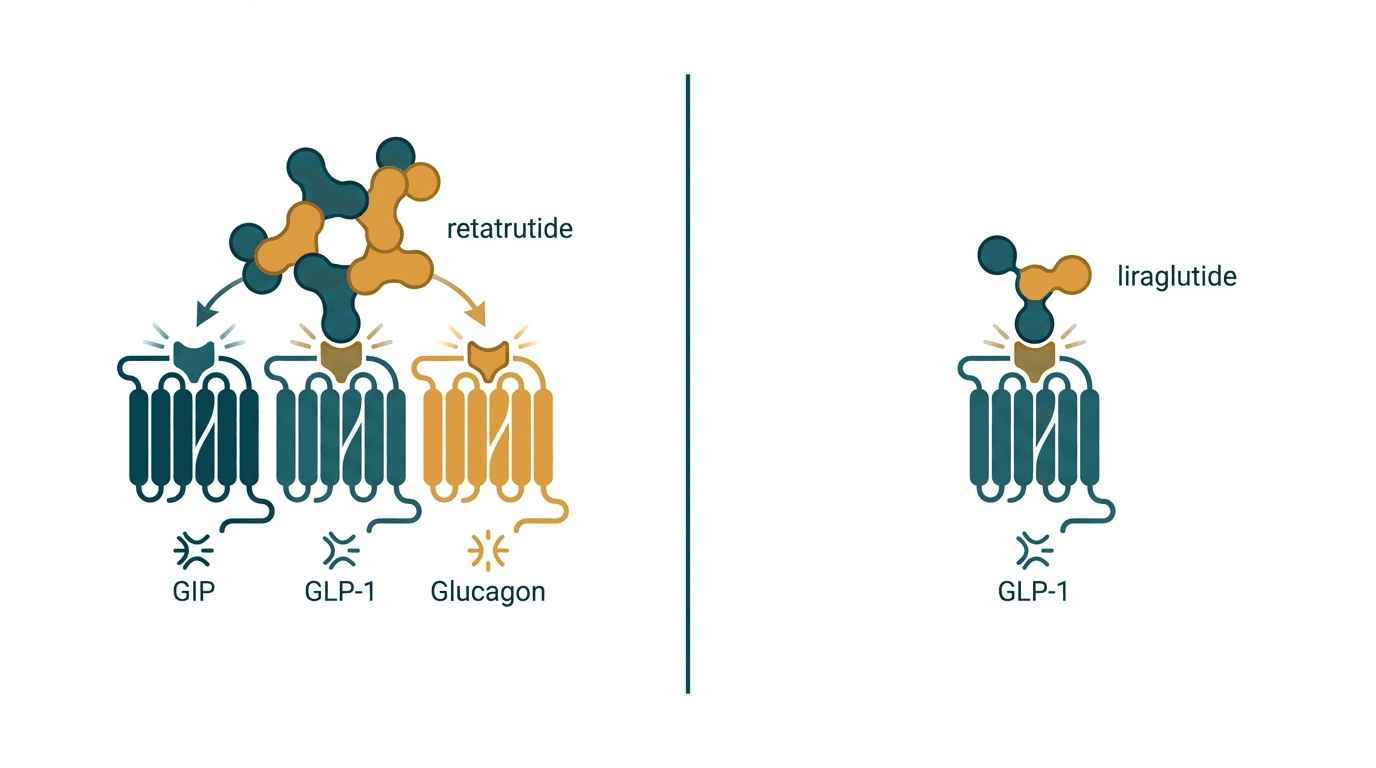
Retatrutide and liraglutide represent two different generations of incretin-based weight loss therapies separated by a massive efficacy gap. Retatrutide is a novel triple agonist targeting GLP-1, GIP, and glucagon receptors simultaneously, producing unprecedented weight loss of up to 24% body weight in Phase 2 trials. Liraglutide (Saxenda/Victoza), a single GLP-1 receptor agonist approved since 2014, achieves a more modest ~8% weight loss. While liraglutide has over a decade of real-world safety data and widespread insurance coverage, retatrutide may redefine the upper limits of pharmacological weight loss once approved.
Head-to-Head Comparison
| Criteria | Retatrutide | Liraglutide |
|---|---|---|
| Primary mechanism | Triple agonist — GLP-1 + GIP + glucagon receptor activation | Single GLP-1 receptor agonist |
| Average weight loss | ~22–24% body weight at 48 weeks (12 mg dose, Phase 2) | ~5–8% body weight at 56 weeks |
| FDA approval status | Phase 3 clinical trials (Eli Lilly); not yet approved | FDA-approved: Victoza (2010, diabetes), Saxenda (2014, obesity) |
| Injection frequency | Once weekly (subcutaneous) | Once daily (subcutaneous) |
| Glucagon receptor activation | Yes — increases energy expenditure and hepatic fat oxidation | No — no glucagon receptor activity |
| GIP receptor activation | Yes — enhances insulin sensitivity and may amplify GLP-1 effects | No — no GIP receptor activity |
| Effect on liver fat (MASLD) | Significant reduction via glucagon-mediated hepatic fat oxidation | Modest reduction (secondary to weight loss) |
| GI side effects | Nausea, diarrhea, vomiting (similar rates to other incretins with slow titration) | Nausea, diarrhea, constipation (well-characterized over 10+ years) |
| Muscle mass preservation | Preliminary data suggests better lean mass preservation (possibly GIP-mediated) | Lean mass loss proportional to total weight loss (~25–40% of weight lost) |
| Cardiovascular data | No completed cardiovascular outcomes trial yet | LEADER trial showed cardiovascular benefit (reduced MACE in T2D) |
| Convenience | Weekly injection — high convenience | Daily injection — lower convenience |
| Approximate monthly cost | Not commercially available yet (estimated $1,000–$1,500 at launch) | $1,300–$1,500/month (Saxenda list price); ~$200–$400 compounded |
When to Choose Each
Choose Retatrutide
Maximum weight loss potential, patients with fatty liver disease (MASLD/MASH), those who prefer weekly dosing, patients inadequately responding to current GLP-1 therapies
Choose Liraglutide
Patients needing an approved therapy now, those with type 2 diabetes and cardiovascular risk, patients wanting established long-term safety data, daily dosing preference for flexible dose adjustment
Verdict
Retatrutide represents a generational leap in obesity pharmacotherapy, delivering roughly three times the weight loss of liraglutide through its triple-receptor mechanism. The addition of glucagon receptor agonism drives increased energy expenditure and liver fat reduction that single GLP-1 agonists cannot match. However, liraglutide remains the pragmatic choice today — it is FDA-approved, widely available, has proven cardiovascular benefits from the LEADER trial, and has 10+ years of real-world safety data. For patients who can wait, retatrutide may obsolete liraglutide for weight management upon approval.
References
- Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-comparator controlled phase 2 trial (2023) — PubMed
- Triple-hormone-receptor agonist retatrutide for obesity — a phase 2 trial (2023) — PubMed
- A randomized controlled trial of 3.0 mg of liraglutide in weight management (SCALE Obesity) (2015) — PubMed
- Liraglutide and cardiovascular outcomes in type 2 diabetes (LEADER trial) (2016) — PubMed
Frequently Asked Questions
How much more weight loss does retatrutide produce compared to liraglutide?
When will retatrutide be available?
Why does retatrutide include a glucagon agonist if glucagon raises blood sugar?
What are the main differences in side effects between retatrutide and liraglutide?
Is retatrutide more cost-effective than liraglutide given the weight loss difference?
Explore next
- Reconstitution CalculatorCalculate exactly how many units to draw on your syringe. Enter your vial size, bacteriostatic water volume, and desired dose.
- Dosage CalculatorFind evidence-based dosing ranges for any peptide. Adjust for body weight, experience level, and administration route.
- Cost CalculatorEstimate peptide costs per dose, per week, per month, and per year. Enter your vial price and dosing schedule to plan your budget.