Semaglutide vs Liraglutide
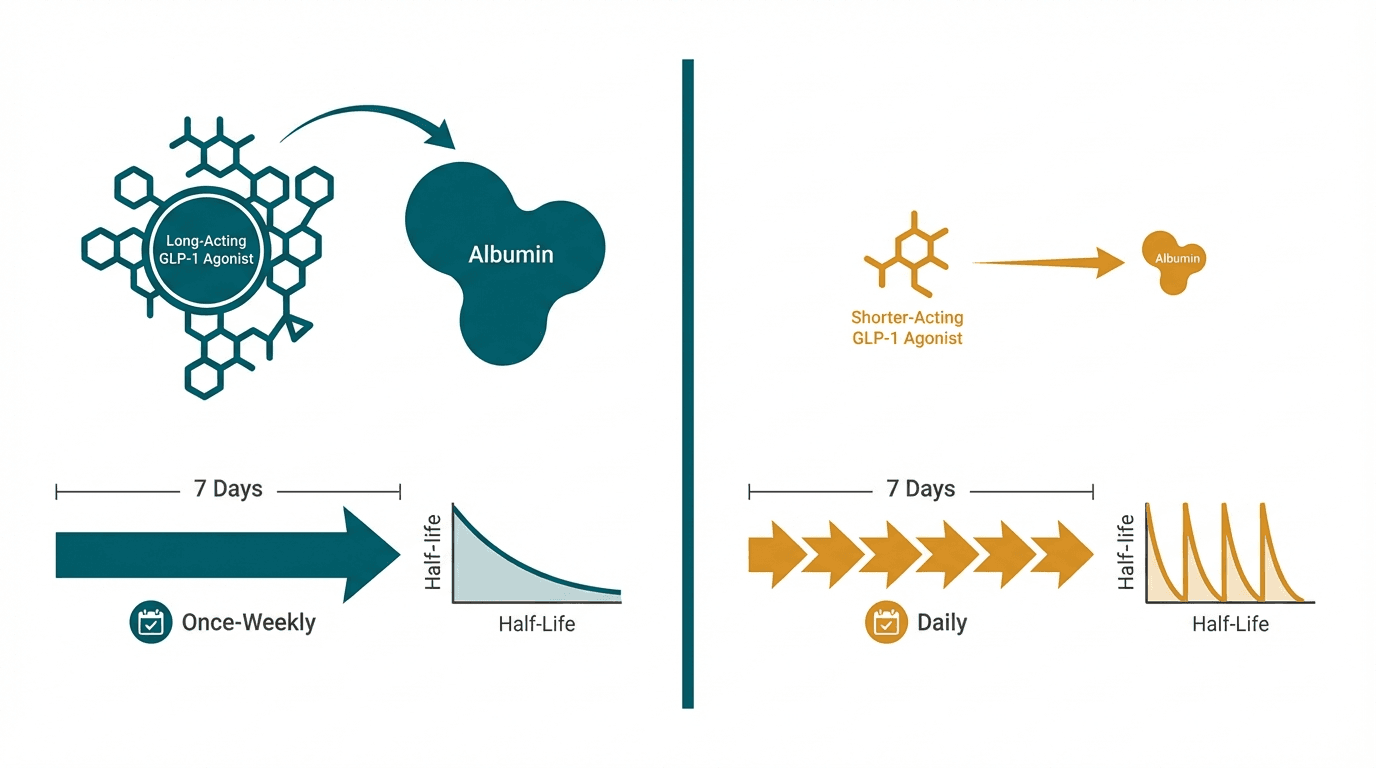
Semaglutide and liraglutide are both GLP-1 receptor agonists made by Novo Nordisk, but they represent a significant generational improvement. Liraglutide (Victoza/Saxenda), approved in 2010, requires daily injections and produces ~8% weight loss. Semaglutide (Ozempic/Wegovy), a structurally optimized successor approved in 2017, achieves ~15% weight loss with once-weekly dosing thanks to superior albumin binding and enzymatic resistance. The STEP 8 head-to-head trial directly confirmed semaglutide's superiority, showing nearly double the weight loss compared to liraglutide.
Head-to-Head Comparison
| Criteria | Semaglutide | Liraglutide |
|---|---|---|
| Primary mechanism | GLP-1 receptor agonist (94% homology to native GLP-1) | GLP-1 receptor agonist (97% homology to native GLP-1) |
| Best for | Adult weight loss, type 2 diabetes, cardiovascular risk reduction | Pediatric obesity (ages 12+), patients wanting a proven first-generation GLP-1 |
| Route of administration | Once-weekly subcutaneous injection or once-daily oral tablet | Once-daily subcutaneous injection |
| Typical dosage | 0.25 mg escalating to 2.4 mg weekly (Wegovy) or 7–14 mg oral daily (Rybelsus) | 0.6 mg escalating to 3.0 mg daily (Saxenda) |
| Average weight loss | ~15.8% at 68 weeks (STEP 8 head-to-head trial) | ~6.4% at 68 weeks (STEP 8 head-to-head trial) |
| Half-life | ~7 days (enables weekly dosing) | ~13 hours (requires daily dosing) |
| Injection frequency | Once weekly (4 injections/month) or daily oral | Once daily (30 injections/month) |
| FDA status | Approved: Wegovy (obesity, 2021), Ozempic (T2D, 2017), Rybelsus (oral, T2D, 2019) | Approved: Saxenda (obesity, 2014), Victoza (T2D, 2010) |
| Cardiovascular outcomes | SELECT trial: 20% MACE reduction in overweight/obese adults | LEADER trial: 13% MACE reduction in T2D patients |
| Side effects | Nausea (44%), vomiting (24%), diarrhea (30%) — side effects more intense at higher efficacy | Nausea (39%), vomiting (16%), diarrhea (21%) — generally milder GI profile |
| Generic/biosimilar availability | No generics available — patent protection through late 2030s | Approaching patent expiry — biosimilars expected in coming years |
| Approximate monthly cost (US list price) | $1,350–$1,430/month (Wegovy) | $1,350–$1,400/month (Saxenda) |
When to Choose Each
Choose Semaglutide
Adults seeking best-in-class single GLP-1 efficacy, patients with cardiovascular disease, anyone preferring weekly injections or oral dosing, first-line treatment for obesity and T2D
Choose Liraglutide
Adolescents aged 12–17, patients wanting the mildest GLP-1 introduction, cost-sensitive patients once biosimilars arrive, or those who did not tolerate semaglutide's more intense side effects
Verdict
Semaglutide is the clear upgrade over liraglutide for most patients. The STEP 8 head-to-head trial demonstrated semaglutide produced 15.8% weight loss versus liraglutide's 6.4% — nearly 2.5 times more effective — with the added convenience of weekly rather than daily injections. Semaglutide also has stronger cardiovascular outcomes data (20% vs 13% MACE reduction). Liraglutide remains valuable for adolescents aged 12+, as a lower-intensity starting option, and will become more accessible as biosimilars arrive. For adult patients choosing between the two, semaglutide is the superior choice by every major clinical measure.
References
- Semaglutide 2.4 mg vs liraglutide 3.0 mg for weight management (STEP 8): a head-to-head randomised trial (2022) — PubMed
- Once-weekly semaglutide in adults with overweight or obesity (STEP 1) (2021) — PubMed
- Liraglutide and cardiovascular outcomes in type 2 diabetes (LEADER) (2016) — PubMed
- Semaglutide and cardiovascular outcomes in patients with overweight or obesity (SELECT) (2023) — PubMed
Frequently Asked Questions
How does the STEP 8 head-to-head trial compare semaglutide and liraglutide?
Is liraglutide safer than semaglutide since it has been available longer?
Both are made by Novo Nordisk — what is structurally different about semaglutide?
Can I take semaglutide orally instead of injecting?
Do semaglutide and liraglutide have the same side effects?
Explore next
- Reconstitution CalculatorCalculate exactly how many units to draw on your syringe. Enter your vial size, bacteriostatic water volume, and desired dose.
- Dosage CalculatorFind evidence-based dosing ranges for any peptide. Adjust for body weight, experience level, and administration route.
- Cost CalculatorEstimate peptide costs per dose, per week, per month, and per year. Enter your vial price and dosing schedule to plan your budget.