Retatrutide vs Semaglutide
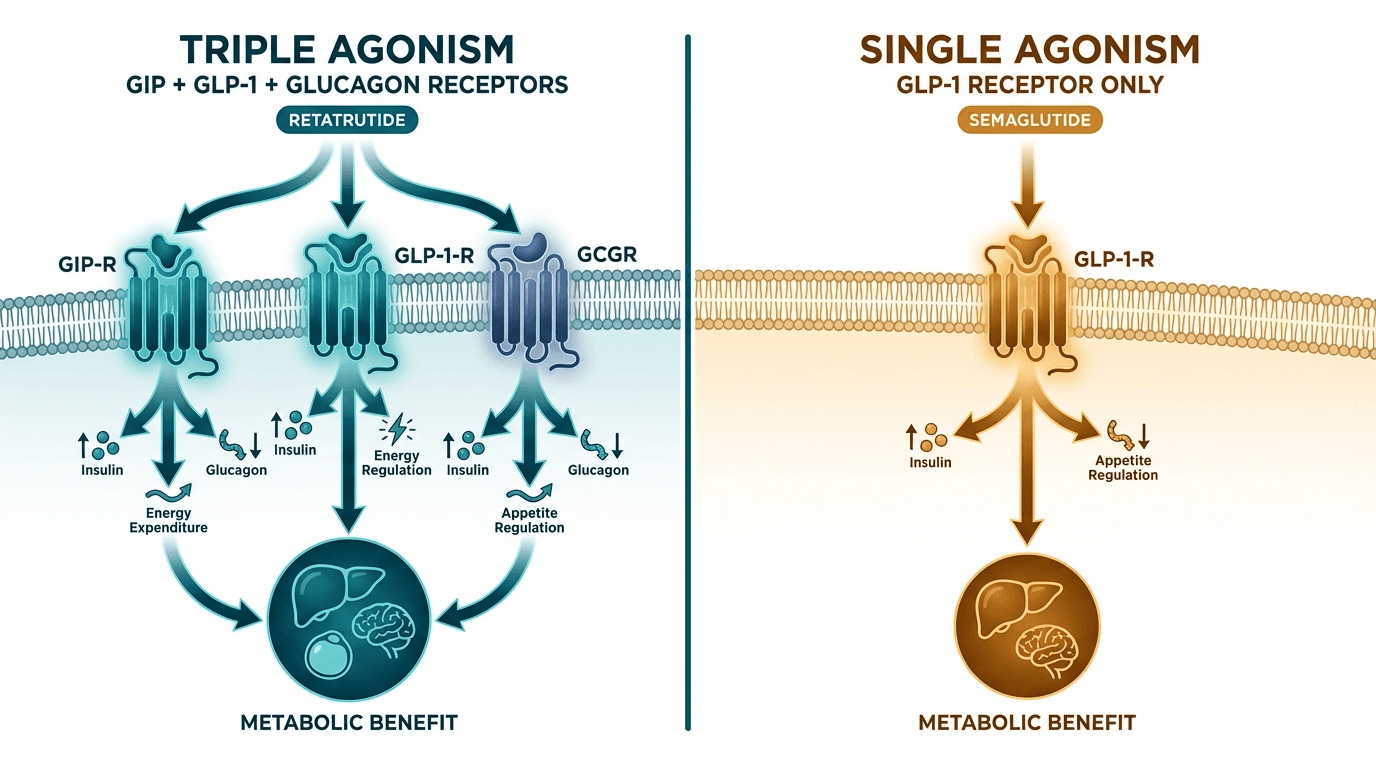
Retatrutide and semaglutide represent two different generations of incretin-based obesity treatments. Semaglutide (Ozempic/Wegovy) is a single GLP-1 receptor agonist that pioneered the modern obesity pharmacotherapy era with ~15% weight loss. Retatrutide (LY3437943) is a triple GLP-1/GIP/glucagon receptor agonist in Phase 3 development that achieved ~24% weight loss in Phase 2 — a roughly 60% improvement over semaglutide. The gap is driven by retatrutide's additional GIP and glucagon receptor activation, which enhance satiety and increase energy expenditure respectively.
Head-to-Head Comparison
| Criteria | Retatrutide | Semaglutide |
|---|---|---|
| Primary mechanism | Triple agonist: GLP-1 + GIP + glucagon receptors | Single GLP-1 receptor agonist |
| Best for | Maximum weight loss, fatty liver disease, metabolic syndrome (once approved) | Weight loss, type 2 diabetes, cardiovascular risk reduction |
| Route of administration | Once-weekly subcutaneous injection | Once-weekly subcutaneous injection (or daily oral tablet for T2D) |
| Typical dosage | 1 mg escalating to 8–12 mg weekly (Phase 2) | 0.25 mg escalating to 2.4 mg weekly (Wegovy) |
| Average weight loss | ~24.2% at 48 weeks (12 mg, Phase 2) | ~15.3% at 68 weeks (2.4 mg, STEP 1) |
| Half-life | ~6 days | ~7 days |
| FDA status | Not approved — Phase 3 trials underway | FDA-approved: Wegovy (obesity, 2021), Ozempic (T2D, 2017) |
| Cardiovascular data | No outcomes data yet | SELECT trial: 20% reduction in major adverse cardiovascular events |
| Effect on liver fat | Dramatic reduction via glucagon-driven hepatic fat oxidation (~86% MASLD resolution) | Moderate reduction in liver fat as secondary benefit of weight loss |
| Side effects | Nausea (26%), diarrhea (22%), vomiting (14%) — Phase 2 | Nausea (44%), vomiting (24%), diarrhea (30%) — STEP 1 |
| Oral formulation | None in development | Available (Rybelsus 7–14 mg daily for T2D) |
| Approximate monthly cost | Not commercially available | $1,350–$1,430/month (brand) |
When to Choose Each
Choose Retatrutide
Future patients seeking maximum weight loss efficacy, individuals with MASLD/fatty liver disease who need hepatic fat reduction, or those who want enhanced metabolic benefits from triple receptor agonism — pending FDA approval
Choose Semaglutide
Patients who need treatment now, those with established cardiovascular disease, patients who prefer oral dosing, or anyone wanting the most-studied GLP-1 agonist with extensive long-term safety data
Verdict
Semaglutide is the proven, FDA-approved standard of care with the longest track record, cardiovascular outcomes data, and an oral option. Retatrutide has the potential to be a significant upgrade, with Phase 2 data showing nearly 60% greater weight loss than semaglutide and notably lower reported GI side effect rates. However, retatrutide is still investigational — Phase 3 results are needed to confirm these findings, and FDA approval is at least a year away. For patients today, semaglutide is the evidence-based choice; retatrutide is the most promising next-generation option on the horizon.
References
- Triple-hormone-receptor agonist retatrutide for obesity — a Phase 2 trial (2023) — PubMed
- Once-weekly semaglutide in adults with overweight or obesity (STEP 1) (2021) — PubMed
- Semaglutide and cardiovascular outcomes in patients with overweight or obesity (SELECT) (2023) — PubMed
- Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-comparator-controlled, parallel-group, phase 2 trial (2023) — PubMed
Frequently Asked Questions
How much more weight loss does retatrutide produce compared to semaglutide?
Why does retatrutide appear to have fewer side effects despite stronger weight loss?
Should I wait for retatrutide instead of starting semaglutide?
Are semaglutide and retatrutide made by the same company?
Will retatrutide preserve more muscle mass during weight loss than semaglutide?
Explore next
- Retatrutide dosage guideRetatrutide dosage guide covering the investigational triple-agonist peptide's dose escalation schedule from Phase 2 trial data, reconstitution instructions, side effect profile, and comparison context with tirzepatide and semaglutide. Educational reference only — retatrutide is not yet FDA approved.
- Semaglutide dosage guideDetailed semaglutide dosage chart covering weight management titration (Wegovy), type 2 diabetes dosing (Ozempic), and oral semaglutide (Rybelsus). Includes compounded reconstitution instructions, side effect profiles, and cycle guidance based on published STEP trial data.