Semaglutide vs Survodutide
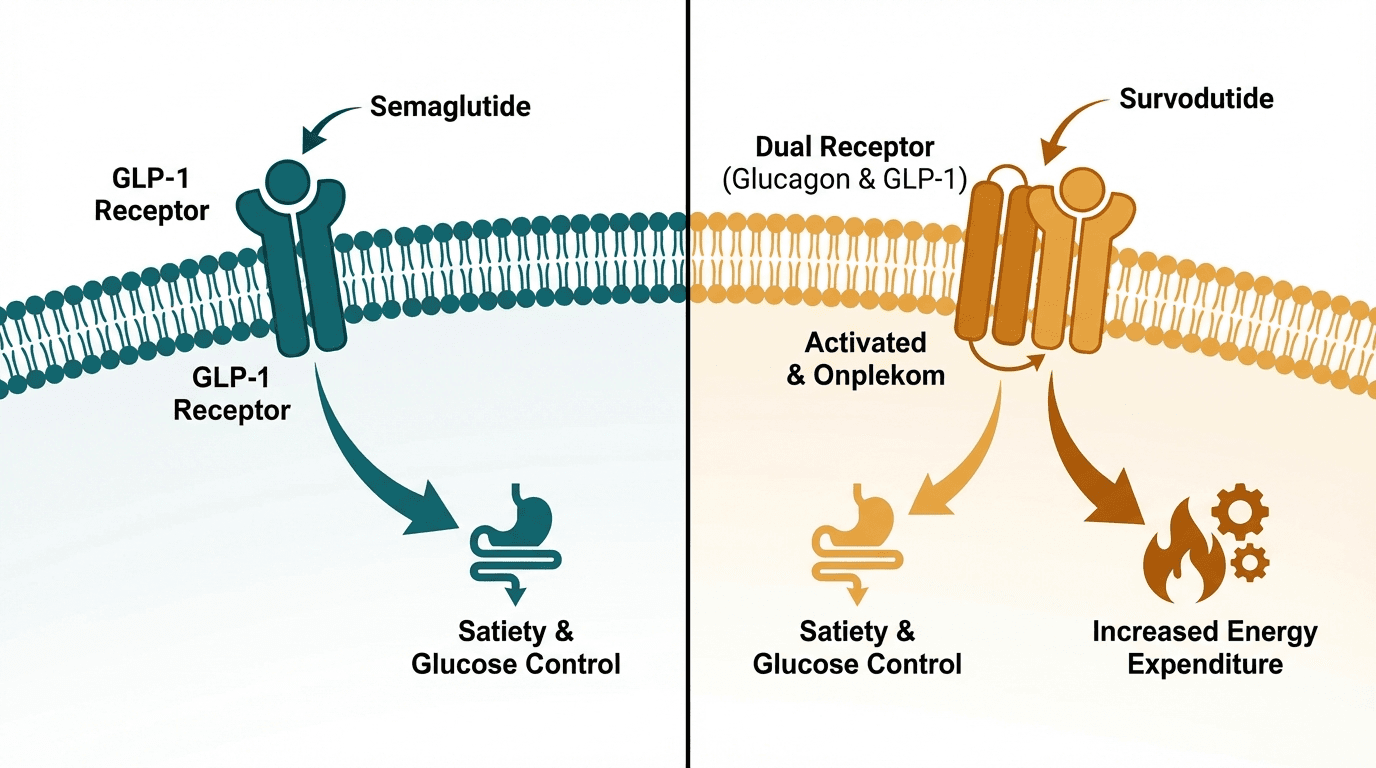
Semaglutide (Wegovy/Ozempic) and survodutide (Boehringer Ingelheim's BI 456906) represent single vs. dual incretin agonist approaches to obesity and metabolic disease. Semaglutide, a pure GLP-1 receptor agonist, is the current gold standard with ~15% weight loss and robust cardiovascular outcomes data (SELECT trial). Survodutide is a dual GLP-1/glucagon receptor agonist in Phase 3 trials showing ~19% weight loss with particularly promising data for liver fat reduction in MASH (metabolic dysfunction-associated steatohepatitis). While semaglutide dominates the current market, survodutide's glucagon component may offer a differentiated profile for patients with fatty liver disease.
Head-to-Head Comparison
| Criteria | Semaglutide | Survodutide |
|---|---|---|
| Primary mechanism | Selective GLP-1 receptor agonist | Dual GLP-1 + glucagon receptor agonist |
| Average weight loss | ~15–17% body weight at 68 weeks (2.4 mg Wegovy, STEP trials) | ~19% body weight at 46 weeks (Phase 2 data, highest dose) |
| FDA approval status | FDA-approved: Ozempic (2017, T2D), Wegovy (2021, obesity), Rybelsus (2019, oral) | Phase 3 clinical trials (Boehringer Ingelheim); not yet approved |
| Injection frequency | Once weekly (subcutaneous) | Once weekly (subcutaneous) |
| Glucagon receptor activation | None | Yes — increases energy expenditure, promotes hepatic fat oxidation |
| Liver fat reduction (MASH/MASLD) | Moderate — secondary to weight loss; some direct hepatic GLP-1 effects | Substantial — glucagon-driven hepatic fat oxidation; up to 87% relative reduction in liver fat in Phase 2 |
| MASH fibrosis improvement | Limited direct evidence for fibrosis reversal | Phase 2 showed MASH resolution in 83% of patients and fibrosis improvement in 52% |
| Cardiovascular outcomes data | SELECT trial: 20% reduction in MACE in overweight/obese adults without diabetes | No completed cardiovascular outcomes trial |
| GI side effects | Nausea (44%), diarrhea (30%), vomiting (24%) — well-characterized | Nausea, diarrhea, vomiting at similar rates; potential for transient hyperglycemia during titration |
| Oral formulation available | Yes — Rybelsus (oral semaglutide, lower efficacy than injectable) | No — injectable only in current development |
| Effect on energy expenditure | Minimal direct effect — weight loss primarily from appetite suppression | Increased resting energy expenditure via glucagon receptor activation (thermogenesis) |
| Approximate monthly cost | $1,350/month (Wegovy list price); $200–$500 compounded | Not commercially available yet |
When to Choose Each
Choose Semaglutide
Proven weight loss therapy now, cardiovascular risk reduction (SELECT trial), patients wanting oral option (Rybelsus), established safety profile, insurance coverage
Choose Survodutide
MASH/MASLD with significant liver fat, patients seeking maximum weight loss, those who need enhanced energy expenditure, patients who failed or plateaued on GLP-1 monotherapy
Verdict
Semaglutide is the proven, available standard with strong cardiovascular outcomes data, multiple formulations (injectable and oral), and years of real-world safety evidence. It remains the first-line choice for most patients seeking pharmacological weight management today. Survodutide is the more compelling option specifically for patients with concurrent MASH/fatty liver disease, where the glucagon receptor component drives dramatic liver fat reduction beyond what GLP-1 alone achieves. If survodutide's Phase 3 results confirm the Phase 2 data, it could carve out a significant niche — or even challenge semaglutide broadly — particularly as liver disease becomes a larger focus of metabolic medicine.
References
- Survodutide (BI 456906), a dual glucagon/GLP-1 receptor agonist, in overweight or obesity: SYNCHRONIZE-1 Phase 2 results (2024) — PubMed
- Once-weekly semaglutide in adults with overweight or obesity (STEP 1) (2021) — PubMed
- Semaglutide and cardiovascular outcomes in patients with overweight or obesity (SELECT trial) (2023) — PubMed
- Survodutide for the treatment of MASH and liver fibrosis: a Phase 2 randomised trial (2024) — PubMed
- Glucagon receptor agonism enhances the metabolic benefits of GLP-1 receptor agonism: a review (2022) — PubMed
Frequently Asked Questions
What advantage does survodutide have over semaglutide for liver disease?
Does the glucagon component in survodutide cause blood sugar problems?
Why not just use semaglutide since it is already available?
When might survodutide become available?
How does survodutide differ from tirzepatide and retatrutide?
Explore next
- Reconstitution CalculatorCalculate exactly how many units to draw on your syringe. Enter your vial size, bacteriostatic water volume, and desired dose.
- Dosage CalculatorFind evidence-based dosing ranges for any peptide. Adjust for body weight, experience level, and administration route.
- Cost CalculatorEstimate peptide costs per dose, per week, per month, and per year. Enter your vial price and dosing schedule to plan your budget.